Abstract:
This article reveals that dysregulation of transcription factor Cra causes excessive glycerol synthesis, a key factor for Escherichia coli growth burden. Overproduced glycerol reduces fructose 1,6-bisphosphate, activating Cra to inhibit glycolysis and promote gluconeogenesis. The Max Planck team designed Cra binding sites in promoters to maintain fructose 1,6-bisphosphate, improving growth and glycerol synthesis. Validated in carotenoid-overexpressing E. coli, this approach shows generality for engineering metabolic pathways, published in *Nature Communications*.
Details:
Project overview
Synthesis metabolism is one of the burdens of engineered bacteria, but its internal mechanism is not clear. The results of this study indicate that the oversynthesis of glycerol caused by the dysregulation of transcription factor Cra activity is an important reason for the growth burden of Escherichia coli. The synthesis of glycerol reduces the level of fructose 1,6-bisphosphate and activates Cra, thereby inhibiting the activity of glycolysis-related enzymes and promoting the activities of gluconeogenesis-related enzymes. Since ATCC cell growth depends on the supply of glucose, inappropriate activation of gluconeogenesis and inhibition of glycolysis may lead to impairment of cell growth under the induction of glycerol pathway. Hannes Link’s team at the Max Planck Institute for Terrestrial Microbiology in Germany designed a Cra binding site in the promoter expressed by the rate-limiting enzyme of the glycerol pathway to maintain a sufficiently high level of fructose 1,6-diphosphate. The authors designed a constitutive set of inducible promoters and oversynthesized carotenoids in E. coli, demonstrating the generalizability of the approach. Related results were published in “Nature communications”.
1. Glycerol oversynthesis leads to growth burden of Escherichia coli
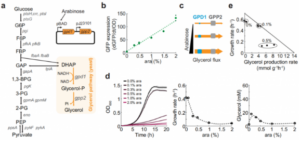
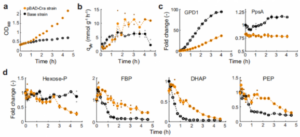
To study how anabolic pathways affect host metabolic processes, the authors overexpressed the glycerol biosynthetic pathway in Escherichia coli. The glycerol pathway is a two-step reaction starting with the glycolytic metabolite dihydroxyacetone phosphate. The first step reaction is catalyzed by glycerol-3-phosphate dehydrogenase (GPD1), which converts dihydroxyacetone phosphate into glycerol-3-phosphate. In the second step, 3-phosphoglycerol phosphohydrolase (GPP2) catalyzes the dephosphorylation of 3-phosphoglycerol to generate glycerol. The E. coli strain used to synthesize glycerol expresses the two genes encoding gpd1 and gpp2 from a plasmid and lacks the glycerol kinase gene (glpK) preventing the utilization of glycerol as a carbon source. Among them, gpd1 is regulated by the pBAD promoter (arabinose inducible), while gpp2 is regulated by the pJ23101 promoter. In the study of growth burden regulation, it was found that the pBAD promoter can linearly promote protein expression. However, the pBAD promoter was unable to regulate growth rate and glycerol synthesis efficiency according to flux balance analysis. However, under the action of glycerol pathway induction, the decrease in growth rate was much more drastic than the flux balance analysis, as shown in Figure 1.
Excessive synthesis of glycerol leads to growth burden of Escherichia coli 2.
Glycerol synthesis activates the transcription factor Cra by reducing the level of fructose 1,6-diphosphate
Metabolome changes were analyzed. A total of 96 metabolites were detected, which remained relatively constant upon induction with 0.1% arabinose but showed strong changes upon induction with 0.5% arabinose. Among them, dihydroxyacetone phosphate (DHAP), the immediate precursor of the glycerol pathway, decreased most strongly when induced by 0.5% arabinose. In addition, dihydroxyacetone phosphate upstream metabolite fructose 1,6-bisphosphate (FBP) was also one of the most significantly changed metabolites. FBP can activate the transcription factor Cra, thereby inhibiting the expression of glycolytic enzyme-related genes and promoting the expression of gluconeogenesis enzyme-related genes. Whether low concentration of FBP can activate Cra to regulate gene expression and enzyme activity. The Cra knockout strain was used as the control group, and 38 proteins in each experimental group were analyzed. It was found that phosphoenolpyruvate synthase (PpsA) was significantly up-regulated in the 0.5% arabinose group, while glyceraldehyde-3-phosphate dehydrogenase (GapA) was significantly up-regulated. ) is significantly reduced. This shows that 0.5% arabinose-induced glycerol pathway can reduce the concentration of FBP to activate Cra, leading to a decrease in GapA activity (glycolysis-related enzymes) and an increase in PpsA activity (gluconeogenesis-related enzymes).
Since the strains were grown in media containing low glucose concentrations, it was hypothesized that activation of gluconeogenesis was an important cause of the growth burden and this was confirmed. Furthermore, the Cra knockout strain grew better than the base strain under high induction of the glycerol pathway. Therefore, Cra can regulate the growth burden of E. coli caused by excessive glycerol synthesis.
Induction of the glycerol pathway activates the transcription factor Cra
3. Metabolic model predicts optimal strategy for glycerol synthesis
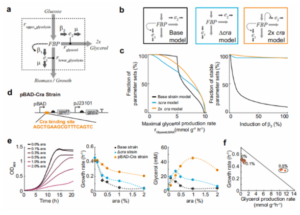
To further demonstrate that Cra transcription is a factor regulating glycerol synthesis, the authors developed a small kinetic model. As shown in Figure 3, the model contains one metabolite (fructose 1,6-bisphosphate) and two enzymes (GapA and GPD1). According to Michaelis-Menten kinetics, fructose 1,6-diphosphate affects the reaction rates of the glycolytic and glycerol pathways. The authors assumed a constant influx in the model and fixed the glycolytic upper limit reaction rate at 4.9 mmol/g/h, implying that fructose 1,6-bisphosphate was synthesized at a constant rate for either glycerol synthesis or biomass synthesis. Three different models (basic model, Δcra model, and 2xcra model) were constructed for analysis. The analysis results showed that the 2xcra model was the best and could obtain high glycerol flux. Therefore, model analysis suggests that regulating Cra to different enzyme activities may increase glycerol synthesis rate.
Theoretical and experimental analysis of the pBAD promoter under the regulation
of Cra to promote glycerol synthesis 4. The pBAD promoter regulated by Cra can improve growth rate and glycerol synthesis.
In the study, it was found that Cra can inhibit the pBAD-Cra promoter, in order to prove the function of the pBAD-Cra promoter , to detect the activities of pBAD promoter and pBAD-Cra promoter in wild-type and Δcra strains. It was found that in the wild-type strain, the activity of the pBAD-Cra promoter was lower than that of the pBAD promoter, indicating that Cra had an inhibitory effect on the promoter. In the Δcra strain, the activity of the pBAD-Cra promoter was slightly higher than that of the pBAD promoter. These results indicate that Cra represses the pBAD-Cra promoter and that this regulation does not function in the absence of Cra. Therefore, the low activity of pBAD-Cra is not only due to the sequence change, but is due to the active inhibition of Cra.
Regulatory effect of Cra on pBAD promoter
5. Cra regulates pBAD promoter to maintain high fructose 1,6-bisphosphate level
under high glycerol flux group changes. As shown in Figure 5, the pBAD-Cra strain could maintain higher fructose-1,6-diphosphate levels at a higher glycerol synthesis rate. This suggests that the interaction between fructose 1,6-diphosphate and Cra counteracts the downward trend of fructose 1,6-diphosphate. First, if the 1,6-diacid fructose level is lower than the critical value, the Cra activity will increase; second, the excessive Cra activity will inhibit the pBAD-Cra promoter activity and inhibit the expression of GPD1; third, the low expression of GDP1 will Restore the original concentration of fructose 1,6-bisphosphate. Therefore, this feedback regulation mechanism not only maintains high levels of fructose 1,6-bisphosphate and increases the rate of glycerol synthesis, but also prevents the metabolic shift from glycolysis to gluconeogenesis in E. coli.
Changes in metabolites and protein content induced by the glycerol pathway
6. Cra regulation promotes the growth of carotenoid-overexpressing Escherichia coli
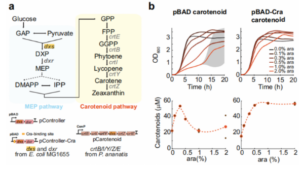
Since many biological substances are synthesized using sugar degradation metabolites as precursors, in order to investigate the universality of the regulation mechanism, The authors used the pBAD promoter to regulate carotenoid synthesis and metabolic pathways (as shown in Figure 6). Carotenoid biosynthesis starts from the glycolytic metabolites pyruvate and glyceraldehyde-3-phosphate, which are converted to farnesyl pyrophosphate via the methylerythritol phosphorylation pathway in Escherichia coli, which is further converted to carotenoids carotene. Two enzymes of the methylerythritol phosphate pathway (Dxs and Dxr) were overexpressed from two pBAD promoters from plasmids called pController and pController-Cra, respectively. Other enzymes of the carotenoid pathway (CrtE/B/I/Y/Z) were expressed from another plasmid (pcarotene) using the native Arabidopsis promoter. The results showed that Cra-regulated promoters could sustain higher growth rates at high induction levels of the synthetic carotenoid pathway, and high inducers did not affect cell growth and production rates. This indicates that the pBAD promoter regulated by Cra has broad universality, and it is possible to regulate other synthetic pathways through glycolytic metabolites.
The Cra-regulated pBAD promoter promotes the synthesis of large amounts of carotenoids
Summary
In this study, the authors used the arabinose-inducible pBAD promoter to regulate the anabolic pathway in Escherichia coli. While the pBAD promoter showed a linear relationship between inducer concentration and expression rate in the case of GFP expression, no glycerol overproduction was observed. In this case, a small increase in the inducer can cause a huge growth burden and a decrease in synthesis efficiency, which eventually leads to a decrease in the concentration of each bioactive component including glycerol. The researchers used metabolomics and proteomics to explore the effect of Cra on pBAD promoter regulation and host metabolic level. From a biotechnological perspective, this method will help to improve strain synthesis technology that can effectively cope with external perturbations such as industrial-scale bioreactors and internal perturbations such as gene expression.
References
Chun-Ying Wang, et al. Metabolome and proteome analyzes reveal transcriptional misregulation in glycolysis of engineered E. coli. Nature Communications . 2021. https://doi.org/10.1038/s41467-021-25142-0.